The resolution of a crystal structure, through any of the methods described above, requires previous knowledge regarding:
- Its approximate chemical composition
- The unit cell dimensions, to be deduced from the diffraction pattern (the reciprocal unit cell),
- The crystal symmetry, to be deduced from the diffraction symmetry (object of this subchapter), and
- The diffracted intensities
The reader can also imagine that the only reliable information we have about symmetry is the one provided by the diffraction pattern, that is, the symmetry of the reciprocal lattice. Therefore, it should now be clear that the problem is to derive the crystal symmetry from the reciprocal lattice symmetry, in the hope that the crystal symmetry will be shown somehow in the diffraction pattern.
FRIEDEL'S LAW
However, there can be some confusion regarding one of the possible symmetry operators in crystals: the centre of symmetry. The existence of the centre of symmetry in the crystal cannot be inferred from the existence of the same operation in the diffraction pattern, because the reciprocal space is (always?) centro-symmetric as was deduced by Georges Friedel (1865-1933), which gave us Friedel's Law.


Left:
A graphic representation of Friedel's Law
in colours that represent the intensities associated with the
reciprocal points around the origin. Equal colours mean identical
intensities.
Right: Friedel's
Law
shown in terms of Bragg's model: reflections produced by
opposite
surfaces of a "mirror" have equal intensities.
Friedel's
Law
states that the
intensities associated with two reciprocal points, given by the indices
(h,
k, l) and (-h, -k, -l),
are almost equal. That is:
This is equivalent to saying that the reciprocal lattice is always centro-symmetric, and thus the "apparent" symmetry of the crystals will be one of the 11 Laue groups.
A pair of reflections such as (h, k, l) and (-h, -k, -l) are known as a Friedel Pair. In the presence of anomalous scattering, these two reflections will show a small difference in intensity, which is very useful to determine the absolute configuration of the molecules and in solving the phase problem through the MAD methodology.
I
(h, k, l) ≈ I (-h, -k, -l)
This is equivalent to saying that the reciprocal lattice is always centro-symmetric, and thus the "apparent" symmetry of the crystals will be one of the 11 Laue groups.
A pair of reflections such as (h, k, l) and (-h, -k, -l) are known as a Friedel Pair. In the presence of anomalous scattering, these two reflections will show a small difference in intensity, which is very useful to determine the absolute configuration of the molecules and in solving the phase problem through the MAD methodology.
As a consequence of Friedel's Law we can state that the crystal can contain lower symmetry than the one displayed by the diffraction pattern. In other words, there will be some type of uncertainty when trying to determine the crystal symmetry from the diffraction experiment.
Fortunately, however, some crystal symmetry operations show their "footprints" in the reciprocal space. For example, some symmetry elements, or in certain lattice types, the arrangement and spacing of lattice planes produces diffractions from certain classes of planes in the structure which are always exactly 180° out of phase producing a phenomenon called systematic extinction. In these cases, certain types of reflections from valid lattice planes (recognizable by simple rules in their hkl indices) will produce no visible diffraction spots.
Systematic absences (or systematic extintions) in hkl reflections arise when symmetry elements containing translational components are present, such as in the following cases:
- lattice centering (translational operations derived from the lattice type),
- screw axes (symmetry axes that imply rotation and an additional translation), and
- glide planes (mirror planes that imply reflection and an additional translation).
The examples below demonstrate how symmetry operations of this type produce zero intensity associated with structure factors whose indices are related by simple rules.
CENTERED LATTICES
Consider a crystal lattice (shown in 2-dimensions), such as the one shown in the figure below (left), with axes a and b. This lattice is called primitive because it contains one lattice node inside the unit cell (actually in terms of 4 quarters of a point).
If for some reason we have interpreted this lattice in terms of another unit cell, with axes A and B (figure on the right), the lattice will become what we call a centered lattice (non primitive) because it contains more than one lattice point inside the unit cell (in this case 2 points: 1/4 in each corner + 1 in the center).
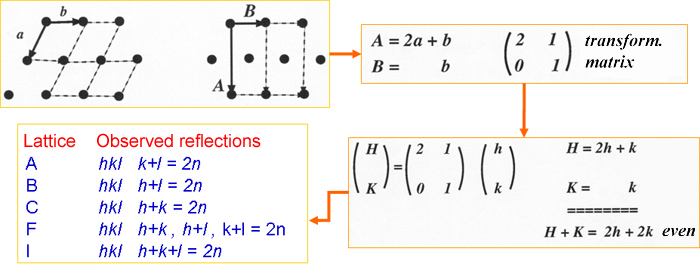
It can be demonstrated that this cell-to-cell transformation matrix can also be applied to the hkl indices of the original lattice to obtain the new HKL indices that, according to the new unit cell, interpret the lattice. If we do this in this example (as is shown in the lower right corner of the figure) we will obtain the equations which relate old and new Miller indices. After adding both equations we will discover that the new Miller indices (HK) are such that its sum (H+K) is always an even number. In other words, if we interpret a diffraction pattern in terms of a reciprocal cell and we only see intensity at those points given by H+K=2n, we can be sure that the crystal lattice is centered (in this particular case a C-centered lattice).
Other systematic absences, which also apply to all hkl reciprocal points, are indicated in the table above.
SCREW AXES
 Two-fold
screw axes, such as the one shown in the figure on the left (a
screw axis parallel to c),
also leave their footprints in the diffraction pattern...
Two-fold
screw axes, such as the one shown in the figure on the left (a
screw axis parallel to c),
also leave their footprints in the diffraction pattern...The reason why such a symmetry operator cancels certain intensities is very simple to deduce if we look at the structure factors that would result from the cooperative scattering of these two atoms:
F
= ƒ
cos 2π
( hx + ky + lz ) +
ƒ cos 2π ( - hx - ky + l
[(1/2) + z] )
However, taking into account the well-known relation:
cos a + cos b = 2
[cos (a+b)/2 ] [cos (a-b)/2]
the formula above can be
rewritten as:F
= 2 ƒ
[cos π (2 l
z + l/2)]
[cos π (2hx
+ 2ky -
l/2)]
This expression vanishes (F=0) for those hkl reflections with h=0, k=0 and l=2n+1. Therefore, diffraction patterns showing systematic absences of this type, or in other words, showing intensity only for reflections of type 00l with l=2n, indicate the existence of a screw axis parallel to the c axis.
Generalizing for other two-fold screw axes, and depending of their direction, the corresponding rules for systematic absences are:
Two-fold
screw axis
Existing
parallel to: reflections
parallel to: reflections
a
h00 h=2n
b
0k0
h=2n
c
00l
h=2n
For systematic absences produced by other screw axis types see the table appearing through this link. See also the International Tables for X-ray Crystallography.
GLIDE PLANES
 Glide
planes, which are
mirror planes
which contain an additional translation (see the figure on the left),
are also responsible for some systematic extinctions in the reciprocal
lattice as seen in the table below:
Glide
planes, which are
mirror planes
which contain an additional translation (see the figure on the left),
are also responsible for some systematic extinctions in the reciprocal
lattice as seen in the table below:Glide
plane
Existing
parallel to: Translation: reflections:
a b/2 0kl k = 2n
a c/2 0kl l = 2n
etc...
For systematic absences produced by other glide plane types see
the table appearing through this link. See also the International Tables for X-ray
Crystallography.parallel to: Translation: reflections:
a b/2 0kl k = 2n
a c/2 0kl l = 2n
etc...
Summarizing: through the observation of systematic extinction rules, such as those shown above, one can confirm the presence of different lattice types (centered lattices) or symmetry elements such as screw axes and glide planes, which provide a very valuable tool for determining the space group (the symmetry) of the crystal.
CENTRE OF SYMMETRY
As discussed in the beginning, there is only uncertainty regarding symmetry elements, and it is the centre of symmetry, as shown by Friedel's Law. However, there are situations where the presence of this symmetry element is fixed by the combination of other symmetry elements whose existence is evident through systematic extinctions.
This is the case of a very frequent space group, P21/c, in which screw axes parallel to the b axis coexist with glide planes perpendicular to b, as shown below:

The symmetry elements shown in the left figure (which relate the positions of the black circles), such as the screw axes parallel to b, and the glide planes (dotted lines) perpendicular to b, are responsible for the systematic absences shown below:
0k0 k =
2n ---> 21
h0l l = 2n ---> c
h0l l = 2n ---> c
The big circles in the left figure, which for instance represent atoms, are repeated by these operators (screw axes and glide planes). But looking carefully at all the big circles, one can conclude that they are also related through centres of symmetry (inversion centres) located on the cell corners (and at half the cell axes), as shown on the right figure as small circles. These inversion centres are thus generated by combining the screw axes and the glide planes, and therefore one can also conclude that P21/c is a centro-symmetric space group.
However, in other cases, making conclusions about the presence or absence of the centre of symmetry requires additional information from:
- some physical measurements (piezoelectric and/or pyroelectric effects)
- statistical tests on the intensity distribution, or
- even the structure solution
But let's go back...
