Crystallization is the process, governed by both thermodynamic and kinetic factors, by which molecules arrange themselves in a natural manner to form a repetitive three-dimensional reticulum we call crystal. The crystallization process consists of two major events: nucleation and crystal growth. Nucleation is the step where the molecules dispersed in the solvent start to gather into clusters, on the nanometer scale (elevating solute concentration in a small region), which becomes stable under the current operating conditions. These stable clusters constitute the nuclei. However when the clusters are not stable, they redissolve. Therefore, the clusters need to reach a critical size in order to become stable nuclei. Such critical size is dictated by the operating conditions (temperature, supersaturation, etc.). It is at the stage of nucleation in which the atoms arrange in a defined and periodic manner which defines the crystal structure — note that "crystal structure" is a special term that refers to the relative arrangement of the atoms, not the macroscopic properties of the crystal (size and shape), although those are a result of the internal crystal structure.
It is not the purpose of these pages to make a comprehensive presentation of the different crystallization techniques which can be found in many textbooks or on different websites, and we will only refer briefly to some of the most common techniques used to obtain protein crystals. However, for beginners we recommend to read the brochure prepared by UNESCO to grow single crystals.
In the case of protein samples, the crystallization experiment begins with a relatively concentrated protein solution (between 2 and 50 mg /ml) to which a reagent solution is added with the intention of reducing its solubility and generating controlled precipitation. Using a gradual increase of concentration and keeping these conditions under control, tiny crystalline nuclei can be obtained which can grow and lead to crystals of adequate size for diffraction experiments (between 0.1 and 0.5 mm).


These generic diagrams show the different areas of a protein-precipitant equilibrium in terms of the concentrations of both components.
Areas shown in yellow (left) and white (right) represent the conditions under which the protein is in solution. The areas depicted in blue represent the conditions under which the protein appears as a precipitate. Both areas are separated by another area (shown in pink) with some supersaturation conditions, suitable for nucleation and crystal growth.
Thus, to maximize the possibility of a successful crystallization experiment, it is necessary to design various experiments, from different starting positions, ie different concentrations of protein and precipitant (arrows of different colours in the diagram on the right).
One of the most common methodologies for these experiments is based on the hanging drop technique.

Scheme of a well reservoir, containing a precipitant solution, capped with a cover slip, as used in the hanging drop technique
The procedure consists, approximately, of the following aspects: A few microliters (1-2 μl) of protein solution are mixed with a more or less equal amount of reservoir solution containing the precipitants (previously balanced with a pH buffer) and are deposited on a cover slip which covers the precipitant reservoir. As the protein/precipitant mixture in the drop is less concentrated than the reservoir solution (normally we mix the protein solution with the reservoir solution at about 1:1), water evaporates from the drop into the reservoir. As a result the concentration of both protein and precipitant in the drop slowly increases, and crystals may form.
On many occasions the sitting drop technique is also used ...
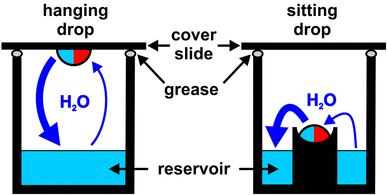
Diagram showing the differences between hanging drop and sitting drop procedures
(taken from doi: 10.1007 / s40828-018-0064-1)
The market offers several types of plates suitable for protein crystallization techniques, as it is shown below. See also the web pages offered by Hampton Research.


Left: A 24 well crystallization plate for protein crystallization using the hanging drop technique
Right: A 24 well crystallization plate for protein crystallization using the sitting drop technique
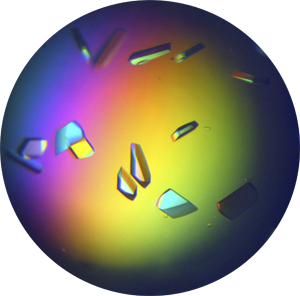

Left: Under the right conditions, crystals can grow in the drop as shown in the picture.
Right: The available crystallization robots can help to prepare very quickly hundreds of drops to find the best initial conditions for crystallization.
Readers interested in the historical development of these crystallization methodologies and their influence on current practice, should consult the article to be found through this link.
The animation below shows the process where lysozyme crystals are growing from an aqueous media. The duration of the process, that takes a few seconds on your screen, corresponds approximately to 30 minutes in real life. This case corresponds to an extremely quick crystal growth process.

With the same enzyme (lysozyme), the video produced by Bernhard Rupp shows the relationship between rapid nucleation and crystal growth rate. The greater the number of nuclei formed, the lower the growth rate, and therefore the smaller the size of the crystals obtained. Compare the size (much smaller) of the crystals growing in the movie below with those shown above.

There is a variety of other techniques available to make crystals grow, such as sitting drops, dialysis buttons, and gel and microbatch techniques.
See also the pictorial library of crystallization drop phenomena offered by Terese Bergfors at the University of Uppsala and take a look to this special issue of Acta Crystallographica.
The advanced reader cannot forget the technical revolution that for X-ray diffraction has recently been introduced, where single-crystal X-ray diffraction ‘snapshots’ are collected from a fully hydrated stream of nanocrystals using femtosecond pulses from a hard-X-ray free-electron laser obtained in a Linac Coherent Light Source. This will probably eliminate most "bottleneck" effects that sometimes crystallization can produce, especially with proteins (see this article published in Nature (2011) 470, 73-77).
But let's go back...
